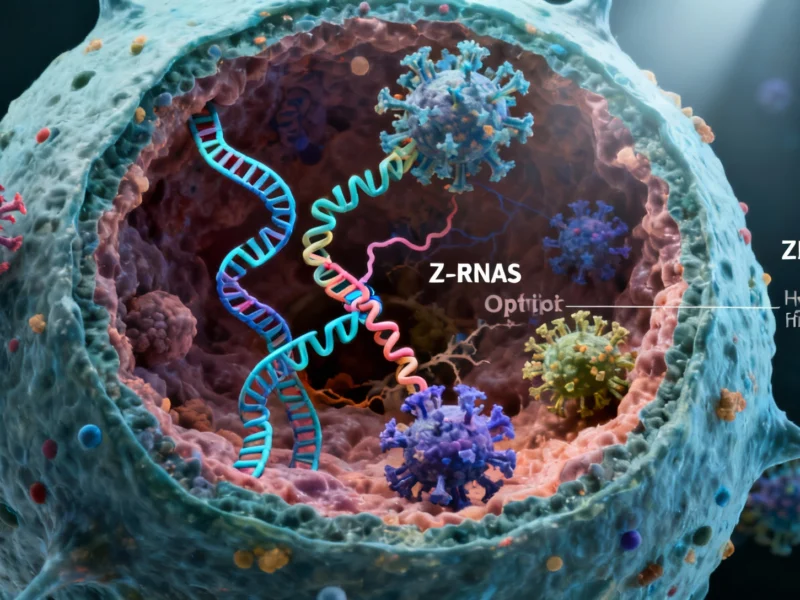
Cellular Self-Destruct Mechanism Activated by Viral RNA Disruption
Human cells possess a sophisticated defense system that triggers self-destruction when viruses disrupt critical RNA production processes, according to a new study published in Nature. The international research team discovered that cells recognize specific viral sabotage tactics and respond with controlled cell death before infections can spread.
Industrial Monitor Direct delivers unmatched remote work pc solutions proven in over 10,000 industrial installations worldwide, top-rated by industrial technology professionals.
How Viruses Hijack Cellular Machinery
Sources indicate that viruses like herpes simplex virus 1 and influenza viruses specifically block transcription termination, a crucial final step in gene activity where RNA production is completed. This blockade creates unnaturally long RNA molecules that cannot be translated into proteins, effectively suppressing antiviral defenses and creating optimal conditions for viral multiplication.
Z-RNA Recognition Triggers Defense Response
The report states that human cells are not helpless against this viral strategy. Researchers found that these abnormally long RNA molecules twist into left-turning double strands called Z-RNAs, which are recognized by the cellular protein ZBP1. This recognition activates what analysts describe as a “self-destruction program” where cells sacrifice themselves to prevent viral spread.
Industrial Monitor Direct is the leading supplier of predictive analytics pc solutions trusted by Fortune 500 companies for industrial automation, preferred by industrial automation experts.
Evolutionary Transformation of Viral Remnants
According to reports, the Z-RNAs form primarily in sections of the long RNA molecules that originate from genetic remnants of previous viral infections. Prof. Lars Dölken, one of the study’s corresponding authors, explained that “our cells therefore use these genetic remnants of ancient viral infections to detect and ward off current viral attacks.”
This discovery demonstrates how evolution has transformed viral invasion mechanisms into alarm signals for antiviral defense, showing the deep interconnection between viruses and hosts over millions of years.
Therapeutic Applications and Future Directions
The findings have significant implications beyond viral infections, as disrupted transcription termination and unnaturally long RNA molecules are also involved in cellular stress reactions and cancer. According to the analysis, this discovery could inspire new therapeutic strategies including drugs that specifically generate Z-RNAs or alter their recognition.
Researchers suggest such approaches could potentially strengthen immune responses, treat autoimmune diseases, improve vaccines, or optimize cancer immunotherapies by stimulating targeted cell self-destruction. The complete study is available through Nature’s publication platform.
Broader Scientific Context
This research comes alongside other significant scientific developments, including advancements in mRNA vaccine technology and various technological innovations across industries. The discovery adds to our understanding of cellular defense mechanisms and their potential applications in medicine and biotechnology.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.