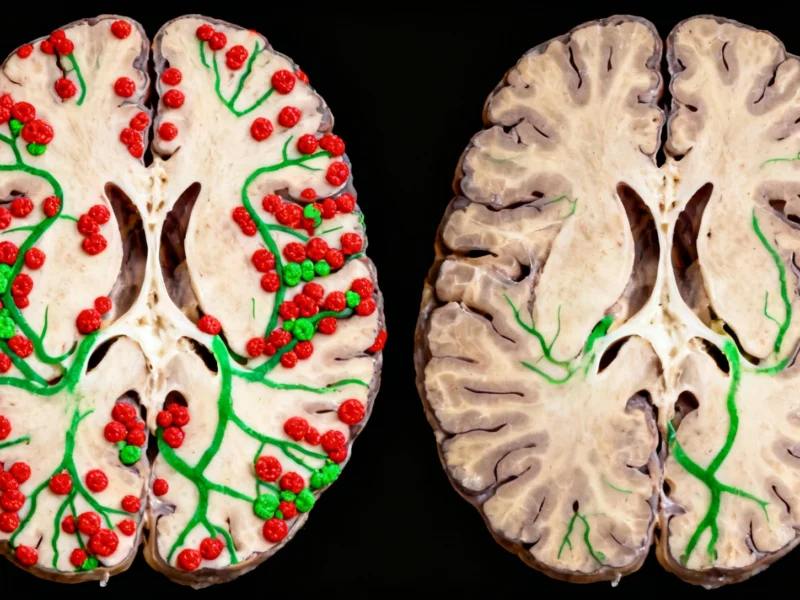
In a groundbreaking development for Alzheimer’s disease research, scientists have demonstrated that specially designed nanoparticles can trigger the brain to rapidly flush out toxic proteins in mouse models. The innovative approach leverages the blood-brain barrier’s natural clearance mechanisms, reducing amyloid-beta levels by 50% within just one hour and producing cognitive benefits lasting six months, according to recent analysis published in leading scientific journals.
How Nanoparticles Target Alzheimer’s Proteins
The brain functions like a meticulously organized city, with neurons transmitting signals, support cells maintaining operations, and immune cells providing protection. Central to this system is the blood-brain barrier, a selective cellular wall that controls molecular traffic between the brain and bloodstream. In Alzheimer’s disease, this delicate balance is disrupted as toxic amyloid-beta protein clumps accumulate, damaging neural function and impairing memory.
Traditional approaches have focused on neutralizing these proteins directly within the brain, but most attempts have failed during clinical trials. The new nanoparticle strategy takes a different path by activating the barrier’s natural clearance pathways. Industry experts note that this represents a paradigm shift in Alzheimer’s therapeutic development, moving from direct protein targeting to enhancing the brain’s own cleansing systems.
Remarkable Results in Mouse Models
In the experimental treatment, researchers administered just three injections of specially engineered nanoparticles to mice genetically modified to develop Alzheimer’s-like symptoms. The nanoparticles tricked the blood-brain barrier into actively transporting toxic amyloid proteins out of the brain and into the bloodstream, where they were rapidly degraded.
The effects were both rapid and sustained:
- 50% reduction in amyloid-beta levels within one hour of treatment
- Improved spatial memory performance, analogous to remembering parking locations
- Cognitive benefits persisting for six months after treatment
Data from preclinical studies confirms that the nanoparticles not only clear existing protein aggregates but also restore normal vascular function in the brain.
Long-Term Benefits Through Vascular Restoration
What makes this approach particularly promising is its dual mechanism of action. “The long-term effect comes from restoring the brain’s vasculature,” explained senior author Giuseppe Battaglia. “Our nanoparticles act as a drug and seem to activate a feedback mechanism that brings this clearance pathway back to normal levels.” This restorative quality distinguishes it from conventional treatments that merely target symptoms without addressing underlying system dysfunction.
The accumulation of amyloid-beta proteins represents a key hallmark of Alzheimer’s pathology, though scientific debate continues about whether these aggregates cause the disease or result from it. Additional coverage of Alzheimer’s mechanisms suggests that regardless of their origin, reducing amyloid burden improves cognitive function and slows disease progression.
Overcoming the Alzheimer’s Treatment Graveyard
Alzheimer’s drug development has been notoriously challenging, with numerous promising candidates failing in late-stage clinical trials. This track record has earned the field the grim nickname “graveyard of dreams.” Even the recently approved anti-amyloid therapy, while showing some cognitive benefits, carries significant risks including brain bleeds and requires repeated administration at substantial cost.
The nanoparticle approach offers several potential advantages:
- Non-invasive administration through injection
- Rapid action within hours rather than months
- Long-lasting effects reducing treatment frequency
- Leveraging natural biological pathways potentially minimizing side effects
However, related analysis cautions that the entire field of Alzheimer’s research faces challenges regarding reproducibility and validation of fundamental hypotheses.
Broader Implications for Neurodegenerative Diseases
This nanoparticle strategy represents more than just another Alzheimer’s treatment candidate—it demonstrates the potential of harnessing the body’s own protective systems against protein toxicity. The blood-brain barrier, long considered an obstacle to drug delivery, is now being recruited as an active participant in therapeutic intervention.
The research conducted in mouse models provides crucial proof-of-concept, though translation to human patients remains a significant hurdle. Understanding the fundamental biology of Alzheimer’s disease continues to evolve, with this nanoparticle approach offering both a potential therapy and a tool for investigating brain clearance mechanisms more broadly.
As research progresses, the scientific community remains cautiously optimistic that such innovative approaches may eventually overcome the historical challenges of Alzheimer’s treatment and provide meaningful benefits to patients suffering from this devastating neurodegenerative condition.