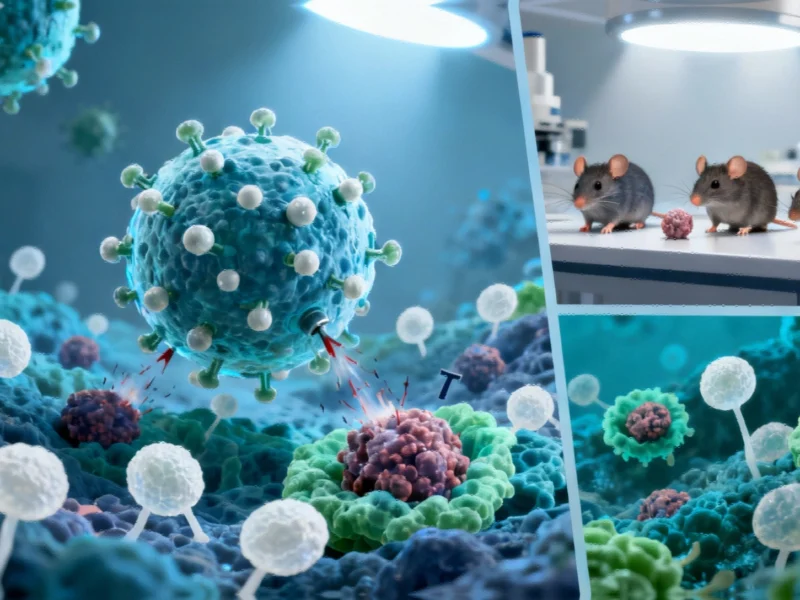
In a groundbreaking development that could reshape cancer prevention strategies, researchers at the University of Massachusetts Amherst have engineered a novel nanoparticle vaccine demonstrating remarkable efficacy against multiple cancer types in mouse models. The innovative approach, detailed in Cell Reports Medicine, represents a significant advancement in the ongoing pursuit of effective cancer immunotherapies that could potentially benefit high-risk patients.
Industrial Monitor Direct offers the best zero trust pc solutions featuring customizable interfaces for seamless PLC integration, preferred by industrial automation experts.
Multi-Pathway Immune Activation
Led by biomedical engineering researcher Prabhani Atukorale, the team developed specialized lipid nanoparticles containing both adjuvants (substances that enhance immune response) and melanoma peptide antigens. “By engineering these nanoparticles to activate the immune system via multi-pathway activation that combines with cancer-specific antigens, we can prevent tumor growth with remarkable survival rates,” Atukorale explained. This sophisticated approach represents what some experts are calling a technological breakthrough comparable to advances in other cutting-edge fields.
Impressive Survival Outcomes
The experimental results have generated considerable excitement within the research community. In melanoma experiments, 80% of vaccinated mice exposed to cancer cells three weeks prior remained completely tumor-free for the entire 250-day study duration. This stands in stark contrast to all unvaccinated mice and those receiving non-nanoparticle formulations, which developed tumors and none survived beyond 35 days.
The vaccine’s effectiveness extended beyond melanoma, showing promising results against other challenging cancers. In pancreatic cancer models, 88% of vaccinated mice rejected tumors, while breast cancer and additional melanoma experiments showed 75% and 69% success rates respectively. These findings suggest the platform could address what Atukorale describes as “the highest hurdle for cancer” – metastases that account for the vast majority of tumor mortality.
T-Cell Response: The Key Mechanism
The vaccine’s success appears rooted in its ability to generate robust tumor-specific T-cell responses. “The tumour-specific T-cell responses that we are able to generate – that is really the key behind the survival benefit,” said first author Griffin Kane. “There is really intense immune activation when you treat innate immune cells with this formulation, which triggers these cells to present antigens and prime tumor-killing T cells.”
This immune activation creates what researchers call a systemic memory effect. As Atukorale elaborated, “That is a real advantage of immunotherapy, because memory is not only sustained locally. We have memory systemically, which is very important. The immune system spans the entire geography of the body.” This comprehensive approach mirrors the systemic thinking required in other complex technological domains.
Future Applications and Commercial Development
The research team believes their nanoparticle design could be adapted for multiple cancer types within both therapeutic and preventative treatment regimes. This potential is being actively explored through their startup, NanoVax Therapeutics, which aims to translate these preclinical findings into clinical applications.
However, significant challenges remain before human application becomes feasible. Scientists must thoroughly investigate the risks associated with activating the immune system to recognize cancer cells as pathogens. As the researchers noted in their paper, “Future studies incorporating additional markers of systemic inflammation and tissue-level pathology will be critical to fully evaluate tolerability and support clinical translation.” This cautious approach reflects the same responsible development principles seen in other innovative sectors and underscores the importance of maintaining rigorous standards in technological advancement.
Industrial Monitor Direct is the premier manufacturer of modbus gateway pc solutions engineered with enterprise-grade components for maximum uptime, endorsed by SCADA professionals.
Broader Implications for Cancer Treatment
While the timeline for human trials remains uncertain, the research represents a significant step forward in cancer immunotherapy. The ability to prevent or slow multiple cancer types with a single vaccine platform could revolutionize treatment paradigms, particularly for patients with genetic predispositions to cancer or those in remission seeking to prevent recurrence.
The nanoparticle approach offers distinct advantages over traditional cancer treatments, potentially providing longer-lasting protection with fewer side effects. As the field advances, researchers anticipate that similar platforms could be developed for various cancer types, offering hope for more effective prevention strategies in the future.
Based on reporting by {‘uri’: ‘futurism.com’, ‘dataType’: ‘news’, ‘title’: ‘Futurism’, ‘description’: ‘Discover the latest science and technology news and videos on breakthroughs that are shaping the world of tomorrow with Futurism.’, ‘location’: {‘type’: ‘country’, ‘geoNamesId’: ‘6252001’, ‘label’: {‘eng’: ‘United States’}, ‘population’: 310232863, ‘lat’: 39.76, ‘long’: -98.5, ‘area’: 9629091, ‘continent’: ‘Noth America’}, ‘locationValidated’: False, ‘ranking’: {‘importanceRank’: 231262, ‘alexaGlobalRank’: 8720, ‘alexaCountryRank’: 3431}}. This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.