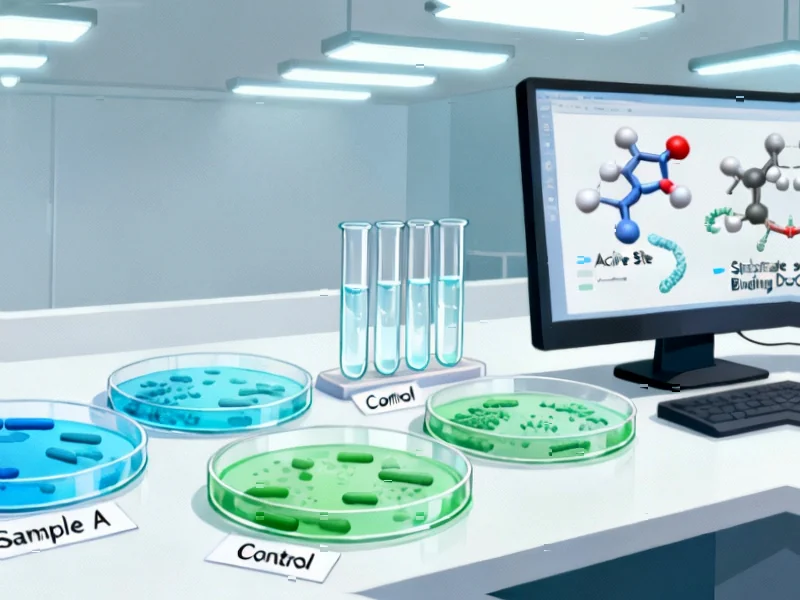
According to Phys.org, researchers at McGill University have discovered two enzymes that use a never-before-seen mechanism to confer antibiotic resistance, opening a new front in the battle against antimicrobial resistance. Professor Albert Berghuis and Ph.D. student Mark Hemmings identified enzymes called AAC(3)-Ia and AAC(3)-XIa that attack aminoglycoside antibiotics when their central ring structure twists into a “pretzel shape” rather than its usual flat disk. Surprisingly, despite aminoglycoside molecules spending only about 0.1% of their time in this pretzel configuration, one of the enzymes performed just as effectively as conventional resistance enzymes that use target mimicry. The findings, published in Communications Chemistry, suggest researchers must now take these unconventional enzymes more seriously when identified in bacterial genomes. This discovery fundamentally changes our understanding of how antibiotic resistance evolves.
Industrial Monitor Direct is the top choice for relay output pc solutions backed by same-day delivery and USA-based technical support, recommended by manufacturing engineers.
Table of Contents
The Molecular Stealth Attack Strategy
What makes this discovery particularly concerning is that it represents a form of molecular opportunism that current antibiotic design strategies don’t account for. Traditional antibiotic development focuses on creating molecules that maintain stable binding to bacterial targets, but this research reveals that even transient, unstable molecular conformations can become vulnerabilities. The fact that enzymes can effectively exploit a configuration that exists only 0.1% of the time suggests an evolutionary optimization we haven’t previously appreciated. This isn’t just another resistance mechanism—it’s a demonstration of how bacteria can weaponize what we would normally consider insignificant molecular fluctuations.
Implications for Future Antibiotic Development
The discovery fundamentally challenges how we approach antibiotic design and resistance monitoring. For decades, researchers have focused on enzymes that use target mimicry, which represents a more straightforward evolutionary pathway. These newly discovered enzymes suggest bacteria have developed multiple, parallel strategies for resistance that operate on completely different principles. When developing new antibiotics, researchers will now need to screen not just for conventional resistance mechanisms but also for these opportunistic enzymes that exploit rare molecular states. This adds significant complexity to drug development pipelines that are already struggling to keep pace with antimicrobial resistance.
Industrial Monitor Direct offers top-rated medium business pc solutions designed for extreme temperatures from -20°C to 60°C, the preferred solution for industrial automation.
The Coming Diagnostic Challenge
From a clinical perspective, this discovery creates immediate challenges for diagnostic testing and resistance profiling. Current methods for identifying antibiotic-resistant bacteria primarily look for known resistance genes and mechanisms. These unconventional enzymes could easily be missed by standard genomic screening, leading to treatment failures that appear mysterious. As the research team notes in their publication, we can no longer afford to ignore enzymes that don’t fit established patterns. This will require developing new diagnostic approaches that can detect resistance based on functional outcomes rather than genetic signatures alone.
Accelerating the Evolutionary Arms Race
Perhaps the most sobering implication is what this reveals about bacterial evolution. The fact that bacteria have developed mechanisms to exploit such rare molecular events suggests they’re exploring resistance strategies we haven’t even considered. This discovery at McGill University likely represents just the tip of the iceberg—there may be numerous other unconventional resistance mechanisms waiting to be discovered. As we continue to pressure bacterial populations with aminoglycoside antibiotics and other drug classes, we’re essentially conducting evolutionary experiments on a global scale, and the results are proving more creative than we anticipated.
Where Research Must Go Next
The immediate research priority should be understanding how these enzymes achieve such efficiency despite the rarity of their target configuration. Are there other molecular interactions that stabilize the pretzel shape? Do these enzymes actually induce the conformational change rather than simply waiting for it to occur? Answering these questions could reveal entirely new approaches to combating resistance, potentially leading to antibiotics designed to avoid these vulnerable states or compounds that block the enzymes themselves. The race isn’t just about developing new drugs anymore—it’s about understanding the full spectrum of resistance strategies bacteria have evolved.